Science That Scales
Our technology platform underpins everything we do
At its core, our FutureGrown™ platform technology is built on the age-old process of fermentation, perfected by years of combined experience from some of the best in the biotech industry. The platform uses biosynthesis to replicate the vast capabilities of nature by producing an individual molecule through precision fermentation to yield a pure, consistent & sustainable functional ingredient. Currently, very few companies have full technical and operational capabilities required to execute the end-to-end precision fermentation solution that Willow can deliver.
Key Technologies and Capabilities to Accelerate Strain Engineering and Process Development
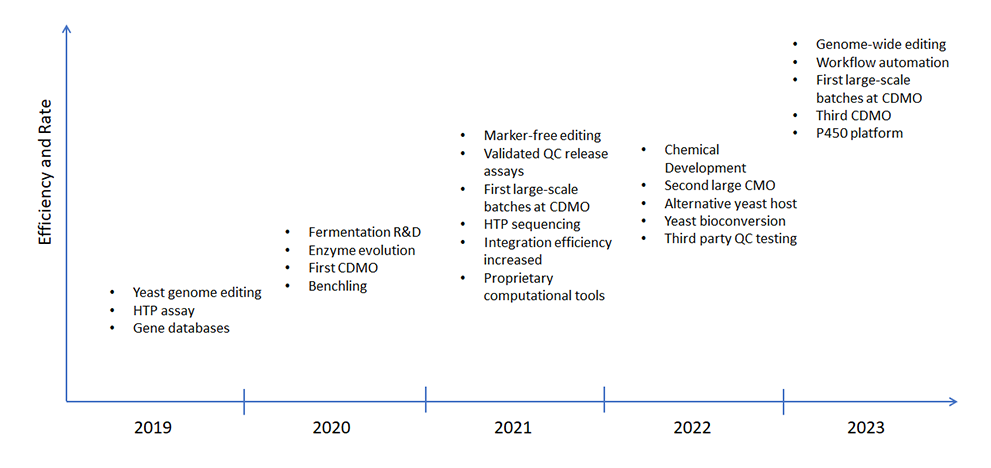
Reliable capacity
We are building to meet big needs – and small too. From kilos to metric tons, our platform provides the ability to scale production to meet the needs of our clients.
Simplified supply chain
The supply chain for our platform technology is far simpler than plant-based production. Manufacturing infrastructure is already in place to meet the demand of clients across the globe.
Trusted team
With extensive experience in industrial biotechnology and an impressive background in launching successful businesses, our team possesses all the tools needed for success.
Industry awarded
More than half of our experienced science and technical team have PhDs and our business team is responsible for cofounding five companies in senior executive roles, which in total sold for over $4 billion.
Computational
Biology
Accelerated Strain
and Enzyme Optimization
Fermentation
(Upstream
Processing)
Chemical
Development
(Downstream
Processing)
Manufacturing
Quality & Regulatory
Computational Biology
At Willow Biosciences, we integrate powerful computational tools at every stage of the Design-Build-Test-Learn (DBTL) cycle, enabling smarter strain engineering and efficient lab operations. This results in rapid decision making, and faster delivery.
- Implementation of a robust information management system to track and manage all R&D operations through one centralized environment
- Advanced design tools for generation of vast yet precise enzyme- and genome-wide libraries in high throughput
- Innovative bioinformatics and data science tools for automated data processing and visualization
- Machine learning tools to train data sets resulting in powerful predictive models that drive engineering efforts
Employing the most advanced technologies in DNA sequencing and DNA synthesis ensures our strains and resulting data are generated quickly with precision and high quality.

Accelerated Strain Optimization
Our Strain Engineering team represents the engine of our technology platform, integrating advanced genetic engineering tools to to engineer enzymes, pathways and entire genomes with precision and speed. The team is responsible for early discovery and characterization of genes and pathways required to produce target active ingredients resulting in the development of proof-of-concept strains. This forms the foundation from which the accelerated strain optimization begins, focused on diversity generation strategies to provide fuel so they can harness the power of recombination in successive rounds of engineering.
- Proprietary genomic databases for gene discovery and characterization
- Rapid automated library design and build capabilities
- Engineering expertise at multiple genetic levels (enzyme, pathway, genome) using cutting-edge gene editing tools
- HTP screening capabilities capable of screening >10,000 strains per week
- NGS and WGS sequencing platforms for sequence analysis and rapid identification of positive genetic edits for recombination
- Work closely with assay team to implement high throughput analytical methods to facilitate screening large libraries
Our strain engineering cycles are rapid and iterative, building and screening through tens of thousands of enzyme variants and strains per week, generating very large and complex data sets that are skillfully mined to drive nature in the direction we need.

Fermentation (Upstream Processing)
- Ensure strain performance is highly conserved across scales (High throughput to lab scale)
- Develop and optimize the production process
- Increase product yields and lower manufacturing costs
Once the process is developed at lab scale, the fermentation team works closely with our manufacturing partners to deliver a commercial process that is robust, scalable, and meets technoeconomic targets.

Chemical Development (Downstream Processing)
Willow Biosciences’ Chemical Development team works closely with the fermentation team to develop integrated, robust processes for isolation and purification of target ingredients , which is essential for development of a viable commercial synthetic biology processes.
- Isolation and purification techniques are designed and developed at bench scale to meet commercial economic targets
- Our team works closely with manufacturing partners to transfer and scale up highly efficient purification processes
Many biotechnology companies focus on strain engineering to drive efficiencies. Willow Biosciences has determined that an additional focus in chemical development leads to a faster, more economical overall process than strain engineering alone with only incremental investment.

Manufacturing
The Willow Biosciences Manufacturing team works closely with our manufacturing partners to ensure a smooth and efficient transition of the developed lab process to large scale-that meets our targets for commercial production. No matter the production level, from 15,000L to 100,000L, Willow Biosciences’ manufacturing partners are experienced in meeting Good Manufacturing Practice (GMP) standards to produce cosmetic, food and nutrition, and pharmaceutical ingredients.

Quality & Regulatory
Willow’s Quality team ensure that products are manufactured in accordance with needed quality standards and are tested against pre-approved specifications using validated and/or verified methods (partners are routinely audited to ensure compliance to those standards). The expectation is that all product inputs used for manufacturing are sourced to meet high quality and that both materials and suppliers, are qualified and approved.
Willow Biosciences Inc. is committed to producing safe and effective products by
- Promoting a quality-oriented culture including managing external regulatory relationships for GRAS, novel foods, pharmaceutical filings etc., as appropriate
- Building Quality by Design into our products to decrease variation in our processes
- Continuous Improvement of our systems, processes, and products to ensure the purity and consistency of Willow’s FutureGrownTM products
The Quality Control team develops, qualifies, and validates methods needed to support our development and manufacturing programs. They ensure methods are suited for their intended purposes during internal development, are transferred in accordance with regulatory requirements, to Contract Manufacturing and/or Contract Testing Organizations as required, support the stability testing program to determine shelf life of the products or intermediates. They ensure partners are monitoring their environments and process utilities to meet industry requirements, ensure and that raw materials are fully released prior to use in manufacturing, and that key performance metrics are met. They also support the stability testing programs to determine shelf life of the products or intermediates.
Manufacturing
The Willow Biosciences Manufacturing team works closely with our manufacturing partners to ensure a smooth and efficient transition of the developed lab process to large-scale that meets our targets for commercial-level production. No matter the production level, from 15,000L to 100,000L, Willow Biosciences’ manufacturing partners are experienced in meeting Good Manufacturing Process (GMP) standards to produce cosmetic, food and nutrition, or pharmaceutical ingredients.